Introduction:DNA damage response (DDR) genes play major role in maintaining the genomic integrity of actively dividing cells such as lymphoid and myeloid cells. Higher rate of mutagenesis and development of cancer is well established in individuals with inherited defects in one of the DDR genes. However, the prevalence of inherited abnormalities in DDR genes in patients with myeloid and lymphoid neoplasms is not well established. Furthermore, the impact of such abnormalities on the clinical course and outcomes of patients with inherited defects in DDR genes is unknown. We investigated the prevalence of germline DDR gene mutations in patients diagnosed with lymphoid neoplasms and compared findings with those in patients diagnosed with myeloid neoplasms.
Method: Tissue and peripheral blood cell-free DNA (cfDNA) from 683 patients with confirmed myeloid or lymphoid neoplasms were tested using a next generation sequencing (NGS) panel of 177 genes. The germline nature of the mutation was confirmed by demonstrating that the mutations were detected in all analyzed DNA in cfDNA. Sequential patients were tested between early 2019 and 2020 without any selection, including newly diagnosed as well as relapsed/refractory patients. The DNA sequencing is based on Single Primer Extension (SPE) library preparation with unique molecular identifier (UMI) (Qiagen, Germantown, MD). Sequencing data of DNA is analyzed using the DRAGEN Platform. Sequence duplicates were removed before calculating VAF.
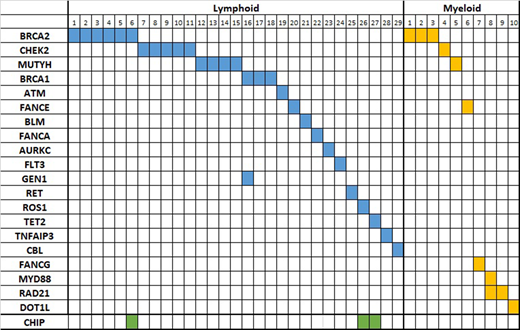
Results:Confirmed germline mutations were detected in the following DNA repair genes: BRCA1, BRCA2, MUTYH, FANCA, FANCE, FANCG, ATM, BLM, and GEN1. In addition germline mutations in MYD88, RET, ROS1 TET2, TNFAIP3, CBL and FLT3 were also detected. Mutations were considered pathogenic only if classified as such in ClinVar database or when showed early termination. Of the 381 patients with various types of lymphoma (follicular, DLBCL, mantle, T-cell lymphoma, marginal zone, and multiple myeloma), 29 (7.6%) showed germline mutations. This is was significantly higher than the prevalence of germline mutations in myeloid neoplasms (P<0.001). In myeloid neoplasms (MDS, AML, and MPN), only 10 patients of 302 had germline mutations (3%). There was no significant difference in age at the time of testing between the two groups (median of 66 years, range 19 to 93 in lymphoid vs median of 65, range 21 to 93 in the myeloid group). We also evaluated the lymphoma group for the prevalence of clonal hematopoiesis of indeterminate potential (CHIP) as defined by the presence of isolated mutations in TET2, DNMT3A, and ASXL1 in cfDNA that were not present in the lymphoma sample. CHIP was detected in 19 of the 352 (5.39%) lymphoma patients without DDR germline abnormalities, while 3 of the 29 with germline mutation (10%) showed CHIP (P=0.2).
Conclusion:The role of DDR mechanism is very significant in lymphomagenesis. Patients with germline mutations in one of the DDR genes should be monitored for the development of lymphoma. However, the presence of a defect in one of the DDR genes does not seem to significantly increase the prevalence of CHIP. The impact of the presence of DDR deficiency on therapy outcome, development of secondary neoplasm needs further investigation.
Donato:Genomics Cooperative:Current equity holder in publicly-traded company.Siegel:Merck:Consultancy, Honoraria, Speakers Bureau;BMS:Consultancy, Honoraria, Speakers Bureau;Janssen:Consultancy, Honoraria, Speakers Bureau;Takeda:Consultancy, Honoraria, Speakers Bureau;Celulatiry:Consultancy;Karyopharma:Consultancy, Honoraria;Amgen:Consultancy, Honoraria, Speakers Bureau.Feldman:Rhizen:Research Funding;Corvus:Research Funding;Kite:Honoraria, Other: Travel expenses, Speakers Bureau;BMS:Consultancy, Honoraria, Research Funding;Viracta:Research Funding;Bayer:Consultancy, Honoraria;Abbvie:Honoraria;Pharmacyclics:Honoraria, Other, Speakers Bureau;Amgen:Research Funding;Cell Medica:Research Funding;Eisai:Research Funding;Kyowa Kirin:Consultancy, Research Funding;Pfizer:Research Funding;Portola:Research Funding;Janssen:Speakers Bureau;AstraZeneca:Consultancy;Trillium:Research Funding;Celgene:Honoraria, Research Funding;Takeda:Honoraria, Other: Travel expenses;Seattle Genetics, Inc.:Consultancy, Honoraria, Other: Travel expenses, Research Funding, Speakers Bureau.Biran:BMS:Consultancy, Honoraria, Other: reimbursement of travel and accommodation, Research Funding, Speakers Bureau;Amgen:Consultancy, Honoraria, Other: reimbursement of travel and accomodation, Research Funding, Speakers Bureau;Janssen:Consultancy, Honoraria, Other: reimbursement of travel and accomodation, Research Funding, Speakers Bureau;Sanofi:Honoraria, Speakers Bureau;KAryopharma:Research Funding.Koprivnikar:Amgen:Speakers Bureau;Novartis:Speakers Bureau;Alexion:Speakers Bureau;BMS:Speakers Bureau.Goy:AbbVie:Research Funding;Morphosys:Research Funding;MD Anderson:Research Funding;Regional Cancer Care Associates/OMI:Current Employment;Infinity Verastem:Research Funding;Xcenda:Consultancy;COTA:Consultancy, Current equity holder in publicly-traded company, Other: leadership role;Kite, a Gilead Company:Consultancy, Current equity holder in publicly-traded company, Honoraria, Other: leadership role, Research Funding;Janssen:Consultancy, Honoraria, Other: leadership role, Research Funding;Celgene:Honoraria, Research Funding;AstraZeneca:Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: leadership role, Research Funding;Acerta:Consultancy, Honoraria, Other: leadership role, Research Funding;RCCA/OMI:Current Employment;PracticeUpdate Oncology:Consultancy;Bayer:Research Funding;CALBG:Research Funding;Constellation:Research Funding;Genentech/Roche:Research Funding;Infinity:Research Funding;Karyopharm:Research Funding;Hackensack UMC and University of Nebraska:Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal